1 Introduction;
Burrowing behavior is commonly found in the invertebrate inhabiting marine soft sediments. The construction of various biogenic structures and their maintenance generate complexity of the habitat (
Reise, 2002;
Kristensen, 2008;
Katrak et al., 2008). The burrow morphology is mostly species specific but in the presence of changes in the physical and biochemical properties of the sediment, the species may modify the structure of burrow to adjust in the changed environment (
Griffis and Suchanek, 1991;
Wolfrath, 1992;
Griffis and Chavez, 1988). Amongst the burrowing species, crabs show significant intra specific variation in the burrow morphology with relation to different environmental and biological factors like sediment composition, vegetation type, shore height, tidal variation, sex and age of the individual (
Takeda and Kurihara, 1987;
Morrisey et al., 1999;
Lim and Diong, 2003;
Chan et al., 2006).
Crab species of genus
Ocypode Weber, 1795 (Decapoda, Ocypodidae) are common inhabitants of sandy shores, mudflats, mangroves and estuarine habitats of tropical and subtropical region (
Dahl, 1953;
Lucrezi et al., 2009;
Trivedi et al., 2015). They are nocturnal feeders and remain in their burrow during day time (Weinstern, 1995). All the
Ocypode species construct semi permanent, deep and complex burrow with circular opening surrounded by sand mounds and feedings marks of the crabs (
Chakrabarti, 1981;
Chan et al., 2006). The burrow provides shelter from harsh environment and predators, and also provides refuge space during molting and mating period of the crab species (
Chakrabarti, 1981;
Brown and Maclachlan, 1990;
Lucrezi et al., 2009). The
Ocypode species were studied extensively for behavior pattern and physiology but studies on burrow morphology are least. According to
Chakrabarty (1981) and
Chan et al. (2006), the ghost crab species produce different kinds of burrow shapes like J shape, Y shape and U shape. The size and shape of the burrows also varies according to the tidal level and shore type.
Ocypode ceratophthalmus is widely distributed ghost crab species in Indo- Pacific region (
Ng et al., 2008). In Gujarat, the species occurs in high abundance on the upper part of sandy shores (
Trivedi et al., 2012;
Trivedi and Vachhrajani, 2012). In the present study, we have correlated the burrow morphology and position of burrow on the sandy shore with the size of the individual. We have also investigated the relationship between the life stages of the individual with the different shapes of the burrow produced by the individual.
2 Materials and Methods
2.1 Study area
The present study was conducted on the exposed sandy shores of two study sites
viz. Sutrapada (20
0 49’ 53" N, 70
0 29’ 17” E) and Kodinar (20
0 45’ 29" N, 70
0 39’ 39” E) located on the Saurashtra coast of Gujarat state, India (
Figure 1). The average width of sandy shores of the study sites was ~20 meters and all shores were steeply (> 2.5° slope) sloping. Both the study sites experience three different seasons like summer (March to June), monsoon (July to October) and winter (November to February) in which summer is hot (42 °C mean air temperature) while winter is cold (25 °C mean air temperature). The tides are semi diurnal with maximum tidal range of 2 meters.
|
Figure 1 Map of Study area. 1. Sutrapada, 2. Kodinar
|
2.2 Sampling methodology
The present study was carried out in the month of March and April, 2014. The burrows were selected randomly in the direction of upper to lower part of sandy shore. The unsaturated resin was poured in the burrows until the burrows were totally filled during the low tide. Crab if, emerged from that burrow was collected and classified according to the sex and the carapace length (CL) was measured using digital vernier calipers (± 0.01 mm; INSIZE Model No. 1137-150). When the burrow cast were solidified (approximately after 1 hour), they were dug out and brought to the lab for further measurement. The casts were cleaned to remove the sediment from the surface. Only complete casts were used for analysis. The burrows were classified according to their shape and various measurements like opening diameter (OD), burrow Inclination angle (IA), total depth (TD), total length (TL), horizontal length (HL), length, diameter and depth of various branches of the burrows and length and width of burrow chambers were measured (
Figure 2). The burrow volume was measured by weighing the burrow (± 1gm) and dividing the burrow weight with the density of the unsaturated resin (0.96 gm/cm
3).
|
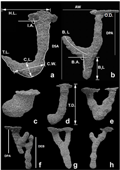
Figure 2 Burrow architecture of Ocypode ceratophthalmus with various measurements of burrow cast. a. J shape, b. J shape with branch at the base, c. Bulb shape, d. Single tube burrow, e. U shape burrow, f. Multi branched burrow, g. Y shape with double opening, h. Y shape burrow. (O. D. - Burrow opening diameter; T. D. – Total depth; T. L. – Total length; I. A.: Burrow inclination angle; H. L. – Horizontal length; C. L. – Chamber length; C. W. – Chamber width; B1L – Branch 1 length; B2L – Branch 2 length; B. A. – Branch inclination angle; DPA – Depth of primary arm; DSA – Depth of secondary arm; DEB- Depth of extra branch)
|
Variation in sediment temperature was measured along the depth of the burrow in the afternoon low tides. Burrows with different opening sizes were selected randomly. The diameter of the burrow openings was measured using vernier calipers (±0.1 mm) and the sand surface temperature at the burrow opening was measured using a digital thermometer (Eurolab ST9269B, ± 0.1°C). The temperature of sediment was measured at every 5 cm depth interval up to the depth of 25 cm. Temperature measurements could not be recorded beyond the depth of 25 cm because of the limited length of probe of the instruments.
2.3 Statistical analysis
The Pearson correlation coefficient was calculated to establish the relationship between carapace lengths of the crab and different morphological parameters of the burrow cast. The mean values of various morphological characters of burrow cast were also compared using paired t test. All the statistical analysis was carried out using PAST statistical package.
3 Results
3.1 Burrow structure
A total of 55 intact burrow casts were obtained of which the host crab was captured for 38 times (28 males and 10 females). Total 8 different types of burrow shapes were recorded which include single tube burrow (15 burrow cast), J shape with branch at the base (12 burrow cast), J shape (7 burrow cast), bulb shape (6 burrow cast), Y shape burrow (4 burrow cast), multi branched burrow (4 burrow cast), Y shape with double opening (4 burrow cast) and U shape burrow (3 burrow cast) (
Figure 2). The crab carapace length showed significant correlation with burrow opening diameter (r = 0.89, p < 0.001) (
Figure 3a), burrow volume (r = 0.55, p < 0.001) (
Figure 3d) and burrow total length (r = 0.57, p < 0.001) (
Figure 3e). The crab carapace length did not show significant correlation with burrow depth (r = 0.24, p = 0.07) (
Figure 3b) and burrow inclination angle (r = 0.03, p = 0.80) (
Figure 3c).
|

Figure 3 New ICT based fertility management model in private dairy farm India as well as abroad
|
Burrow type called Y shape with double opening (YDO) (Figure 2g) (n = 4) had largest volume (465.42 ± 266.93 cm3) with an average burrow opening diameter of 24.54 ± 4.80 mm. The YDO shaped burrows were constructed by the crabs with mean carapace length of 16.85 ± 5.67mm (n = 2). The burrow was vertical with mean inclination angle of 90.25 ± 18.02°. The inclination angle of primary arm (90.25 ± 18.02°) was significantly smaller than the secondary arm (97.05 ± 17.76°; paired t test, t = 2.35, df = 3, p<0.05). The primary and secondary arm of the burrow joined together in the straight shaft and the straight shaft ended with small chamber at the base. Both the primary and secondary arms of the burrow opened on the surface and the burrow opening diameter of primary arm (24.54 ± 4.80 mm) was significantly larger than the secondary arm (19.08 ± 0.89 mm; paired t test, t = 4.31, df = 3, p<0.05). The total depth of the burrow was 36.22 ± 12.80 cm. Depth of primary arm (16.25 ± 9.25 cm) was slightly but significantly higher than depth of secondary arm (15.6 ± 9.42 cm; paired t test, t = 2.91, df = 3, p<0.05). Total mean length of the burrow was 42.1 ± 14.48 cm and the horizontal distance between burrow openings of both the arms was 13.77 ± 4.34 cm. The chamber situated on the base had length of 6.15 ± 2.14 cm and width of 4.12 ± 1.55 cm.
U shape burrows (Figure 2e) (n = 3) had mean burrow volume of (399.39 ± 236.42 cm3) with an average burrow opening diameter of 22.47 ± 5.39 mm. The U shaped burrows were constructed by the crabs with mean carapace length of 14.02 ± 4.52 mm (n = 2). The burrow was vertical with mean inclination angle of 98.00 ± 28.58°. The inclination angle of primary arm (98.00 ± 28.58°) was larger than the secondary arm (97.33 ± 5.85°; paired t test, t = 2.91, df = 2, p = 0.48). Both the primary and secondary arms of the burrow opened on the surface and the burrow opening diameter of primary arm (22.47 ± 5.39 mm) was significantly larger than the secondary arm (16.86 ± 1.19 mm; paired t test, t = 2.91, df = 2, p<0.01). The total depth of the burrow was 20.26 ± 6.59 cm. The depth of primary and secondary arm was similar. Total mean length of the burrow was 33.63 ± 8.95 cm and the horizontal distance between burrow openings of both the arms was 16.52 ± 2.64 cm.
J shape with branch at the base (JBB) (Figure 2b) (n = 12) had mean burrow volume of 386.40 ± 259.94 cm3 with an average opening diameter of 18.73 ± 5.40 mm. The burrows were constructed by the crabs with mean carapace length of 12.37 ± 4.77 mm (n=9). The burrow was vertical in shape with mean inclination angle of 93.16 ± 18.58° and depth of 21.83 ± 7.82 cm. The main shaft takes turn towards the surface in the form of branch which does not extend to the surface and ends in the forms of spherical blind end. Second branch is formed from the base of the main shaft and ends making small chamber at the base. The mean length of the burrow was 25.81 ± 8.09 cm. The length of branch 1 (11.31 ± 3.19 cm) (Side branch) was significantly higher than the branch 2 (8.07 ± 3.19 cm; paired t test, t = 1.79, df = 11, p<0.001) (Base branch). The depth of primary arm (14.3 ± 5.45 cm) was significantly higher than the depth of secondary arm (7.55 ± 2.62 cm; paired t test, t = 1.80, df = 11, p<0.0001). The inclination angle of primary arm (109.41 ± 20.35°) was significantly higher than the main shaft (paired t test, t = 1.83, df = 11, p<0.001). In 70 percent burrows, the primary arm faced the seaward orientation. The horizontal distance between main arm and primary arm was 11.57 ± 5.14 cm.
Bulb shaped (Figure 2c) (n = 6) were the smallest burrow in terms of depth with mean depth of 12.29 ± 3.47 cm. The burrows were vertical in shape with mean inclination angle of 95.08 ± 17.31° and mean burrow volume of 376.97 ± 240.80 cm3. The mean burrow opening diameter was 19.07 ± 5.02 mm and they were constructed by the crabs with mean carapace length of 12.29 ± 3.45 mm (n = 4). In the bulb shape burrows, the burrow opens directly in a large chamber. The mean length and width of the chamber were 6.43± 1.71 cm and 5.46 ± 1.78 cm, respectively. The mean total length of the burrow was 23.72 ± 9.37 cm.
The single tube burrow (ST) (
Figure 2d) (n =15) had mean volume of 366.22 ± 236.19 cm
3 with mean opening diameter of 18.14 ± 5.07 mm. The burrows were constructed by the crab having mean carapace length of 11.66 ± 3.69 mm (n =10). The burrow inclined vertically from the surface with the mean inclination angle of 93.11 ± 17.08° and ended with chamber (CL: 3.06 ± 0.59 cm CW: 4.48 ± 0.53 cm) at the base. The mean depth and length of the burrows were 19.22 ± 7.50 cm and 23.39 ± 8.85 cm, respectively. The mean horizontal distance of the burrow was 3.13 ± 0.60 cm.
The multi branch burrows (MB) (
Figure 2f) (n = 4) had mean volume of 365.06 ± 248.30 cm
3 with mean opening diameter of 19.50 ± 5.16 mm. The MB burrows looked similar to JBB shape burrows, but they all had an extra branch attached to the base of the secondary branch. In MB burrow except primary arm no other branch reach the surface and they all ended with spherical blind lobe. The extra branch attached with secondary branch base had multiple chambers. The burrows were constructed by the crab having mean carapace length of 12.82 ± 4.32 mm (n=3). The burrows inclined vertically with mean inclination angle of 96.63 ± 22.19°. The burrow had mean depth of 26.25 ± 7.99 cm in which the mean depth of extra branch (15± 4.10 cm) was significantly higher than the mean depth of primary arm (11.25 ± 4.03 cm; paired t test, t = 2.35, df = 3, p<0.01). The total mean length of the burrow was 31.08 ± 8.91 cm and the horizontal distance of the burrow was 8.09± 8.91 cm.
The J shaped burrows (J) (
Figure 2a) (n = 7) had mean burrow volume of 215.32 ± 110.08 cm
3 with an average burrow opening diameter of 14.43 ± 4.61 mm. The burrows were vertically inclined with mean inclination angle of 98.65 ± 15.88°. The J shaped burrows were constructed by crab with mean carapace length of 8.87 ± 3.41mm (n = 5). The mean depth and length of the burrow were 14.11 ± 5.88 cm and 24.30 ± 8.03 cm respectively. The horizontal length of the burrow was 12.11 ± 2.22 cm. The mean length and width of the chamber situated on the base was 2.32 ± 1.04 cm and 6.24 ± 2.28 cm.
The Y shaped burrows (Y) (
Figure 2h) (n = 4) had mean volume of 265.81 ± 101.20 cm
3 with an average opening diameter of 15.25 ± 3.93 mm. The burrows were constructed by crabs with mean carapace length of 9.69 ± 3.45 mm (n = 3). The primary and secondary arm of the burrows joined the main shaft and ended with small chamber at the base. The burrows were inclined down with an average inclination angle of 88.64 ± 11.04°. In all the burrow casts obtained, the secondary branch did not reach the surface and ended up with blind spherical end. The mean depth of the burrow was 26.25 ± 7.21 cm. The depth of the primary arm (9.0 ± 2.68 cm) was significantly longer than the secondary arm (6.9 ± 2.14 cm; paired t test, t = 2.38, df = 3, p<0.01). The mean horizontal distance of the burrow was 4.82 ± 3.14 cm. Total mean length of the burrow was 24.67 ± 9.12 cm. The chamber attached at the base had length of 2.44 ± 1.16 cm and width of 3.82 ± 1.02 cm.
3.2 Vertical temperature profile of burrows
The depth wise variation in burrow temperature was studied for various burrow shapes recorded in the present study. Results revealed similar pattern in temperature variation for all the burrow shapes. The sand surface temperature recorded was 42-45°C which remained similar for all the burrow types. The temperature declined to 33 °C at a depth of 5 cm. After the depth of 5 cm the rate of temperature drop decreased to 1 to 1.5°C at every 5 cm. The temperature recorded at the deepest part of the burrow that could be measured up to 25 cm was 28-29.5°C, which was 14-15.5°C cooler than the surface temperature (
Figure 4).
4 Discussion
In the present study, the burrow morphology of brachyuran crab
Ocypode ceratophthalmus burrows showed variation in size, shape and complexity of structure ranging from single tube structure with single opening and no branches to multi branched burrows with multiple opening. The size of the crab species is measured in terms of carapace length. The carapace length affects the burrow opening size because crabs walk sideways direction. In the present study, the crab carapace length showed strong correlation with burrow opening diameter, burrow volume and burrow length which showed that the larger crabs had greater burrow diameter, larger burrow volume and larger depth as compared to medium sized and juvenile crabs.
Lim (2006) found similar kind of relationship of crab- carapace size with burrow opening in brachyuran crab
Uca annulipes and
Uca vocans. She suggested that the burrow of
U. vocans were larger in size as compared to
U. annulipes because
U. vocans is larger species as compared to the other species and it requires larger burrow opening which enables the species to move comfortably inside the burrow.
8 different shapes like single tube burrow, J shape with branch at the base, J shape, Bulb shape, Y shape burrow, multi branched burrow, Y shape with double opening and U shape burrow were recorded for burrow architecture of
Ocypode ceratophthalma.
Chan et al. (2006) have studied the burrow architecture of
Ocypode ceratophthalmus on the sandy shores of Hong Kong and have reported presence of five different shapes of burrow which includes J shape burrow, J shape with branch at the base burrow, Y shape burrow, spiral burrow and single tube burrow. In the present study, more complexity is recorded in the structure of
O. ceratophthalmus burrows as compared to previous studies (
Chakrabarti, 1981;
Chan et al., 2006). In the present study, differences in the burrow architecture were reported with the age and size of crab. Juvenile crab had made J shape, Y shape and single tube burrows, adult crabs had created JBB shape, bulb shape, MB shape while older and large crab have made YDO and U shape burrows
|

Figure 4 New ICT based fertility management model in private dairy farm India as well as abroad
|
The burrow types like J shape, Y shape and single tube burrows made by juvenile crabs were shallow in depth with narrower opening diameter and lesser volume. Chakrabarti (1981) and Chan et al. (2006) have also observed similar pattern in juveniles of the species, as noted by us. They stated that the juveniles of the species have smaller gill area and because of that they cannot tolerate prolonged air or sunlight exposure without renewing their respiratory water. In this situation, the juveniles have to leave their burrow frequently to go to sea water to renew their respiratory water.
The single tube burrows and bulb shaped burrows were created by adult and juvenile of the species. Similar kinds of burrows were also reported in other brachyuran crab species like
Uca pugilator, Cradisoma carnifex and
Macrophthalmus parvimanus. These kinds of burrows are believed to be temporary and made by the species to get refuge during the high tide time or get protection from the predator (
Braithwaite and Talbot, 1972;
Christy, 1982). In the present study, the juveniles of the species have created J shape burrows with mean depth of (14 cm to 19 cm) which are little shallower than J shape burrows reported for juveniles of
O. ceratophthalmus and
O. quadrata in different countries like India, Taiwan, America and Hong Kong. (Chakrabarti, 1981; Takahashi, 1932; Hill and Hunter, 1973;
Chan et al., 2006). In Hong Kong, the juveniles of
O. ceratophthalmus make J shape burrow with branch at the base (
Chan et al., 2006) but in the present study this particular burrow type was reported to be made by the adults of the species.
In Y shape burrows, two types of burrows were reported. First type is the simple Y shape in which burrow opening is available on primary arm while the second type is YDO in which the opening is available on both arms. In both types the orientation of the primary arm was reported towards seaward side while the orientation of the secondary arm was reported towards landward sides.
Chan et al. (2006) and
Chakrabarti (1981) have reported similar kind of pattern in terms of arm orientation in Y shape burrow made by
O. ceratophthalmus on the sandy shore of Hong Kong and Bay of Bengal of India. In the present study, the secondary arm of the Y shape burrow did not reach the surface but ended with spherical blunt end.
Hill and Hunter (1973) also reported similar pattern in Y shape burrows made by the individuals of
O. quadrata on Texas coast. In the present study, the adults of
O. ceratophthalmus had created YDO burrow type in which the secondary arm opens on the surface. The diameter of secondary arm was significantly smaller than the primary arm and inclination angle of primary arm was significantly smaller than the primary arm. YDO type of the burrow is first time reported for
O. ceratophthalmus in present study and it has not been reported even for other crab species.
The function of the secondary branch of Y shape burrows is still unknown but it has been proposed that the secondary arm may provide shelter to the crab individuals from the splash of the waves and predators (
Chakrabarti, 1981). In the present study, the YDO burrows ended with small chamber at the base but the size of the chamber was smaller as compared to the chamber size reported in Y shape burrows made by individuals of
O. ceratophthalmus in Hong Kong (
Chan et al., 2006). Such kind of chamber was not observed in the Y shape burrows of crab species in other studies (
Takahashi, 1932;
Hill and Hunter, 1973;
Chakrabarti, 1981). In fiddler crab males of the species make such kind of chambers during the mating season (
Lim, 2006;
Christy, 1982) so it may be believed that presence of such chambers in
O. ceratophthalmus burrows may provide protection against predators or shelter during mating season. In the present study, multi branched burrows (MB) were also recorded. Such kinds of burrows were also recorded in
O. ceratophthalmus population of Bay of Bengal but not found in Hong Kong population (
Chakrabarti, 1981;
Chan et al., 2006).
Chan et al. (2006) have quoted the reason behind the absence of multi branched burrow in
O. ceratophthalmus population in Hong Kong. They have stated that the study had been carried out in maximum population abundance zone of the species but not in the backshore zone where the individuals may have created multi branch burrows. In the present study U shape burrows had both the branches opening on the surface. Such kinds of burrows were created by large and older crabs. The U shaped burrows were not reported in previous studied on the species (
Chakrabarti, 1981;
Chan et al., 2006).
Specific pattern was observed in the distribution of different burrow shapes from upper to lower part of the sandy shore. Maximum number of J shape, Y shape, and single tube shaped burrows which are occupied by juveniles were recorded in the lower part of sandy shore near the water line while burrows containing other shapes like JBB shape, Bulb shaped, MB shape, YDO and U shape burrows occupied by adult and older individuals were located on the upper part of the sandy shore away from the water line. The adult individuals of the species have bigger gill surface area which helps them to tolerate the exposure of air or sunlight for longer period of time. Because of the above mentioned adaptation, the larger individuals of the species stay in the burrow for entire day and come out on the surface only in night time for feeding. This adaptation also helped them to reduce the risk of predators. It was also observed that in the burrow shapes made by adults, the lower part of the burrows contain moisture which help the individuals to respire without changing their respiratory water.
The burrow temperature variation followed specific pattern. During the study period the sediment surface temperature recorded was 42-45°C which could be lethal for the species but the sediment temperature dropped along with the depth of the burrow (sub surface). The temperature recorded in the deepest part of the of the burrow was near 29°C and the temperature reduced up to 14-15°C which suggests that the burrows can provide refuge or shelter to the crabs during the sunny days. Similar kind of pattern of temperature gradient was also observed in burrows of Hong Kong population of
O. ceratophthalmus (
Chan et al., 2006).
Ferrow (1971) had stated that Y shaped burrows are utilized by females while, males utilize spiral shape burrows but such kind of pattern of shape partition of burrows between male and females was not observed in the present study as well as in the other studies on burrows of
O. ceratophthalmus (
Chakrabarti, 1981;
Chan et al., 2006).
The individuals of O. ceratophthalmus population living on the sandy shores of Saurashtra coast, Gujarat have created different shapes of burrows which are highly influenced by their body size and different life stages. The burrows serve different functions in the life of an individual and further studies should be carried out to investigate the effect of different seasons and sediment type on the burrow morphology of the species.
Acknowledgements
The authors are thankful to the Ministry of Earth Science, Govt. of India for financial support under the head of project entitled “Studies on Brachyuran Crabs of Saurashtra Coast” (Grant No. MoES/16/06/2013-RDEAS dated 11.11.2014). The authors are also thankful to Mr. Gunjan M. Soni, Ms. Dhruva Trivedi, Ms. Barkha Purohit, Mr. Vishal Pankhania and Mr. Kalpesh Gohel for technical assistance.
Brown A.C. and McLachlan A., 1990, Ecology of sandy shores. Elsevier Publishers. Amsterdam pp. 328.
Chakrabarti A., 1981, Burrow pattern of Ocypode ceratophthalma (Pallas) and their environmental significance. Journal of Paleontology, 187:113–130.
Chan K.K.B., Chan K.K.Y. and Leung P.C.M., 2006, Burrow architecture of the ghost crab Ocypode ceratophthalma on a sandy shore in Hong Kong. Hydrobiologia, 560:43–49.
Christy J.H., 1982, Burrow structure and use in the sand fiddler crab, Uca pugilator (Bosc). Animal Behaviour, 31:687–694.
Dahl E., 1952, Some aspects of the ecology and zonation of the fauna on sandy beaches. Oikos, 4:1–27.
Farrow G.E., 1971, Back reef and lagoonal environment of Aldabra Atoll, distinguished by their crustacean burrows. In Stoddart, D. R. and C. M. Yonge (eds), Regional Variation in Indian Ocean Coral Reefs. Proceedings of the Symposium of the Zoological Society of London, pp. 455–500
Griffis R.B. and Chavez F.L., 1988, Effects of sediment type on burrows of Callianassa californiensis Dana and C. gigas Dana. Journal of Experimental Marine Biology Ecology, 117:239–253.
Griffis R.B. and Suchanek T.H., 1991, A model of burrow architecture and trophic modes in thalassinidean shrimp (Decapoda: Thalassinidea). Marine Ecology Progress Series, 79:171–183.
Hill G.W. and Hunter R.E., 1973, Burrows of the ghost crab Ocypode quadrata (Facricius) on the Barrier Islands, south central Texas coast. Journal of Sediment Petrology, 43:24–30.
Katrak G., Dittmann S. and Seuront L., 2008, Spatial variation in burrow morphology of the mud shore crab Helograpsus haswellianus (Brachyura, Grapsidae) in South Australian saltmarshes. Marine and Freshwater Research, 59:902–911.
Kristensen E., 2008, Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. Journal of Sea Research, 59:30–43.
Lim S.S.L. and Diong C.H., 2003, Burrow-morphological characters of the fiddler crab, Uca annulipes (H. Milne Edwards, 1837) and ecological correlates in a lagoonal beach on Pulau Hantu, Singapore. Crustaceana, 76:1055–1069.
Lim S.S.L., 2006, Fiddler crab burrow morphology: How do burrow dimensions and bio turbative activities compare in sympatric populations of Uca vocans (Linnaeus, 1758) and U. annulipes (H. Milne Edwards, 1837). Crustacean, 79:525–540.
Lucrezi S., Schlacher T.A. and Simon W., 2009, Monitoring human impacts on sandy shore ecosystems: a test of ghost crabs (Ocypode spp.) as biological indicators on an urban beach. Environmental Monitoring and Assessment, 152:413–424.
Morrisey D.J., Dewitt T.H., Roper D.S. and Williamson R.B., 1999, Variation in the depth and morphology of burrows of the mud crab Helice crassa among different types of intertidal sediments in New Zealand. Marine Ecology Progress Series, 182:231–242.
Ng P.K.L., Guinot D. and Davie P.J.F., 2008, Syestema brachyuroram part 1. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology, 17:1–286.
Reise K., 2002, Sediment mediated species interaction in coastal waters. Journal of Sea Research, 48:127–141.
Takahashi S., 1932, On the burrows of Ocypode ceratophthalma Fabricius. Kwagaku, 2:329–335 (in Japanese).
Takeda S. and Kurihara Y., 1987, The distribution and abundance of Helice tridens De Haan (Crustacea, Brachyura) burrows and substratum conditions in a north eastern Japan salt marsh. Journal of Experimental Marine Biology Ecology, 107:9–19.
Trivedi J.N., Gadhvi M.K. and Vachhrajani K.D., 2012, Diversity and habitat preference of brachyuran crabs in Gulf of Kutch, Gujarat, India. Arthopods, 1(1): 13�–23.
Trivedi J.N. and Vachhrajani K.D., 2012, Distribution and diversity of brachyuran crabs along the coastal region of Junagadh district, Gujarat. Proceedings of National Seminar on Biodiversity and Conservation of Coastal and Marine Ecosystems of India (BNHS, IUCN, MFE), pp 8–14.
Trivedi D.J., Trivedi J.N., Soni G.M., Purohit B.D. and Vachhrajani K.D., 2015, Crustacean fauna of Gujarat state of India: A review. Electronic Journal of Environmental Science, 8:23–31.
Weinstern R.B., 1995, Locomotor behavior of nocturnal ghost crab on the beach: focal animal sampling and instantaneous velocity from three dimensional motion analysis. Journal of Experimental Biology, 98:989�–999.
Wolfrath B.,1992, Burrowing of the fiddler crab, Uca tangeri, in the Ria Formosa in Portugal and its influence on sediment structure. Marine Ecology Progress Series, 85:237–243.

 Author
Author  Correspondence author
Correspondence author






